Vitamin B12 deficiency severely disrupts your bone marrow’s ability to produce healthy blood cells, leading to megaloblastic anemia where abnormally large, ineffective red blood cells form instead of normal ones. You’ll also experience problems with white blood cell development, causing hypersegmented neutrophils and compromised immune function, plus platelet formation issues resulting in thrombocytopenia. These changes occur because B12 is essential for DNA synthesis during cell division. Understanding the complete recovery timeline and monitoring strategies can help you recognize early warning signs.
Understanding Vitamin B12’s Role in Bone Marrow Function

Megaloblasts—abnormally large, immature red blood cells—signal that your bone marrow isn’t functioning properly when vitamin B12 runs low. Your body needs this vitamin to synthesize DNA in red blood cell precursors. Without adequate B12, cell division becomes impaired, creating the characteristic megaloblastic changes that define megaloblastic anemia.
When vitamin B12 deficiency strikes, your bone marrow responds with hyperplastic megaloblastic erythropoiesis. This means you’re producing more immature red blood cell precursors, but they can’t mature correctly. The result? Larger, oval-shaped megaloblasts that can’t carry oxygen effectively.
You’ll also see disrupted hematopoiesis affecting other blood cells. Giant metamyelocytes and hypersegmented neutrophils appear in your bloodstream, indicating widespread bone marrow dysfunction that extends beyond just red blood cells.
How B12 Deficiency Disrupts Red Blood Cell Production
When vitamin B12 becomes scarce in your system, DNA synthesis breaks down at the cellular level, triggering a cascade of problems in red blood cell development. Your bone marrow starts producing abnormally large, oval-shaped cells called megaloblasts instead of healthy red blood cells.
This vitamin deficiency creates asynchronous development where your cell nucleus and cytoplasm mature at different rates. You’ll experience hyperplastic megaloblastic erythropoiesis – increased red cell precursors without corresponding mature cells.
| Normal Development | B12 Deficiency Effects |
|---|---|
| Synchronized nucleus/cytoplasm | Asynchronous maturation |
| Normal-sized cells | Giant metamyelocytes |
| Single-nucleus erythroblasts | Multinucleated erythroblasts |
| Adequate mature RBCs | Reduced functional cells |
| Normal MCV levels | Elevated MCV |
This disruption leads to megaloblastic anemia, characterized by reduced hemoglobin levels and elevated mean corpuscular volume in your blood work.
Impact on White Blood Cell Development and Immune Function
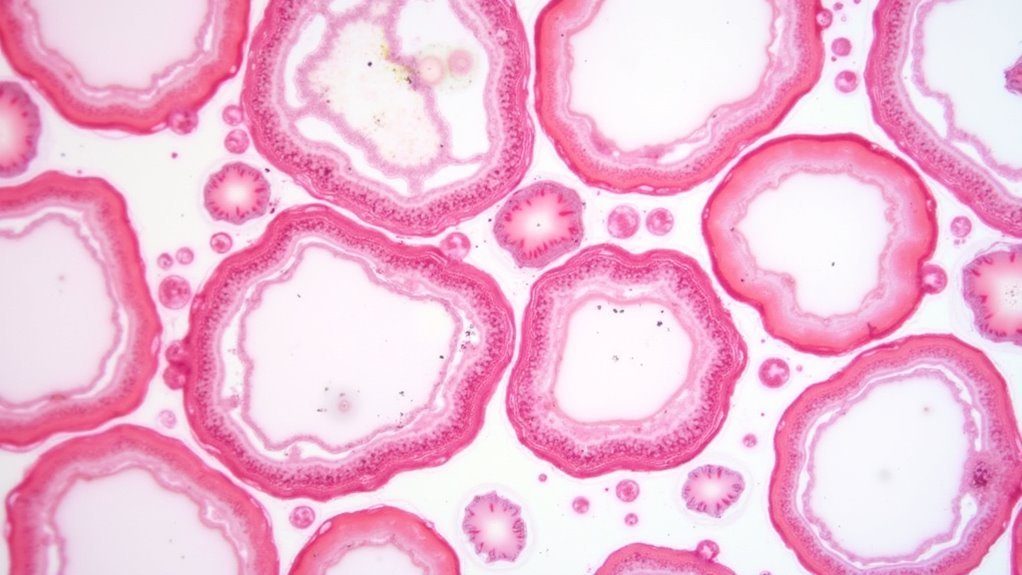
While red blood cells bear the brunt of B12 deficiency’s effects, your white blood cells don’t escape unscathed. When you’re deficient in B12, your bone marrow can’t synthesize DNA properly, disrupting white blood cell development and causing leukopenia—a dangerously low white blood cell count.
This deficiency creates hypersegmented neutrophils, abnormal cells that appear in your blood smears as clear markers of megaloblastic anemia.
Your immune function takes a serious hit during B12 deficiency. With fewer functional white blood cells circulating in your blood, your body struggles to fight off infections effectively. You’ll find yourself more susceptible to pathogens as your compromised immune system can’t mount adequate responses.
Fortunately, B12 supplementation restores your white blood cell counts within weeks, rebuilding your immune defenses.
Platelet Formation Problems in B12-Deficient Bone Marrow
Beyond white blood cells, your platelets also suffer significant damage when B12 deficiency disrupts your bone marrow’s cellular production. Your megakaryocytes can’t mature properly, leading to thrombocytopenia and reduced platelet formation. This creates dangerous bleeding risks throughout your body.
When megaloblastic anemia develops from B12 deficiency, your bone marrow shows telltale signs of dysfunction:
- Giant metamyelocytes and abnormal megakaryocytes with polylobated nuclei appear
- Myeloid-to-erythroid ratios become disrupted, reducing mature platelet counts
- Large, immature platelets form that don’t function properly
Your body experiences ineffective thrombopoiesis, where megakaryocyte precursors fail to produce adequate platelets.
These compromised platelets can’t perform their clotting functions effectively, further increasing your hemorrhagic complications risk. B12 deficiency fundamentally sabotages your entire platelet production system.
Megaloblastic Changes in Bone Marrow Cells

When you’re deficient in B12, your bone marrow cells undergo dramatic megaloblastic changes that disrupt normal blood cell development.
You’ll find abnormally large erythroblasts with nuclear-cytoplasmic maturation defects, along with giant metamyelocytes that shouldn’t exist in healthy marrow.
These changes also produce the characteristic hypersegmented neutrophils you’ll see in peripheral blood, reflecting the profound impact B12 deficiency has on cellular DNA synthesis and division.
Abnormal Cell Size Changes
If you examine bone marrow samples from patients with B12 deficiency, you’ll notice striking abnormalities in cell size and structure that reveal the profound impact of impaired DNA synthesis.
The most prominent changes include:
- Megaloblastic erythropoiesis – You’ll observe abnormally large red cell precursors that can’t complete normal maturation due to disrupted DNA synthesis.
- Oval macrocytic cells – These develop instead of healthy red blood cells, creating the characteristic enlarged, oval-shaped appearance.
- Multinucleated erythroblasts – These aberrant cells form when nuclear division becomes impaired while cytoplasmic development continues.
You’ll also find giant metamyelocytes in the myeloid lineage, demonstrating that B12 deficiency affects multiple cell types.
The myeloid-to-erythroid ratio often becomes reversed as erythroid hyperplasia compensates for ineffective cell production.
Hypersegmented Neutrophil Formation
Among the most diagnostically significant findings in B12 deficiency, hypersegmented neutrophils stand out as a hallmark feature that you’ll consistently observe in peripheral blood smears. These abnormal neutrophils contain five or more nuclear lobes, reflecting impaired DNA synthesis characteristic of megaloblastic anemia.
| Feature | Normal Neutrophils | Hypersegmented Neutrophils |
|---|---|---|
| Nuclear Lobes | 2-4 lobes | 5+ lobes |
| Cell Size | Standard | Enlarged |
| DNA Synthesis | Normal | Impaired |
When vitamin B12 deficiency occurs, your bone marrow’s granulocyte precursors develop megaloblastic features, including disproportionate nuclear and cytoplasmic development. This leads to ineffective hematopoiesis and the characteristic hypersegmented appearance. You’ll find these cells serve as critical diagnostic markers, providing direct insight into the underlying pathophysiology of impaired DNA replication and abnormal cell division processes.
Erythropoiesis Maturation Defects
Beyond the peripheral blood findings like hypersegmented neutrophils, B12 deficiency creates profound disruptions within the bone marrow itself, where erythropoiesis undergoes characteristic megaloblastic transformation.
Your bone marrow struggles to make normal red blood cells due to impaired DNA synthesis, resulting in megaloblastic anemia with distinctive features.
The maturation defects you’ll observe include:
- Asynchronous nuclear-cytoplasmic development – creating larger-than-normal erythroid precursors with oval-shaped cells
- Hyperplastic erythropoiesis – featuring multinucleated erythroblasts and giant metamyelocytes throughout the marrow
- Elevated mean corpuscular volume – producing macrocytic red blood cells that reflect the underlying synthesis problems
Your myeloid-to-erythroid ratio often decreases due to excessive erythroid hyperplasia, while megakaryocytes develop polylobated nuclei with characteristic “salt-and-pepper” chromatin patterns.
Pancytopenia: When All Blood Cell Lines Are Affected
When vitamin B12 deficiency reaches severe levels, it doesn’t discriminate between blood cell types—it affects them all. You’ll develop pancytopenia, where your red blood cells, white blood cells, and platelets simultaneously drop to dangerously low levels. This condition signals that megaloblastic anemia has progressed beyond just affecting red blood cells.
Your bone marrow becomes hyperplastic yet ineffective, producing enlarged precursor cells that can’t mature properly. You’ll notice macrocytosis and hypersegmented neutrophils in blood tests, classic markers of vitamin B12 deficiency.
Your lactate dehydrogenase levels will spike, reflecting increased cell breakdown and turnover.
The good news? Once you start B12 treatment, your blood counts typically recover within weeks, demonstrating how reversible this serious condition can be.
Bone Marrow Aspirate Findings in B12 Deficiency
While blood tests reveal the surface effects of vitamin B12 deficiency, examining your bone marrow aspirate discloses the underlying chaos within your body’s blood cell factory.
Your bone marrow aspirate shows hyperplastic megaloblastic erythropoiesis, meaning it’s working overtime producing abnormal red blood cells. The megaloblastic anemia creates distinctive morphological changes that pathologists can easily identify:
- Multinucleated erythroblasts – abnormal red blood cell precursors with multiple nuclei
- Giant metamyelocytes – oversized white blood cell precursors indicating disrupted maturation
- Asynchronous nuclear-cytoplasmic development – cell components maturing at different rates
You’ll notice larger-than-normal red cell precursors throughout the aspirate.
Fortunately, these changes don’t indicate malignancy or other blood disorders – they’re specifically caused by your B12 deficiency disrupting normal cellular development processes.
Laboratory Markers Indicating Bone Marrow Dysfunction
You’ll notice that B12 deficiency creates distinct laboratory markers that signal bone marrow dysfunction.
Your lab results will typically show dramatically elevated LDH levels, sometimes reaching 5160 U/L, which reflects the increased cell turnover and hemolysis occurring in your system.
You’ll also see hypersegmented neutrophils on your peripheral blood smear, a hallmark finding that indicates impaired DNA synthesis due to insufficient B12.
Elevated LDH Levels
Lactate dehydrogenase (LDH) levels skyrocket in vitamin B12 deficiency, serving as a critical laboratory marker that reveals the extent of bone marrow dysfunction occurring beneath the surface.
When your B12 stores become depleted, elevated lactate dehydrogenase levels can reach alarming heights of 5160 U/L, signaling massive cellular destruction within your bone marrow.
This enzyme elevation directly correlates with the severity of megaloblastic anemia you’re experiencing. Your bone marrow struggles with ineffective erythropoiesis, producing oversized, immature red blood cells that can’t effectively transport oxygen throughout your body.
Key indicators of B12-related bone marrow dysfunction include:
- Dramatically increased LDH levels reflecting tissue damage
- Hemolysis occurring during defective cell production
- Poor-quality red blood cells with reduced functionality
Hypersegmented Neutrophil Presence
How can laboratory technicians spot the telltale signs of B12 deficiency lurking in your blood cells? They’ll search for hypersegmented neutrophils—abnormal white blood cells displaying five or more nuclear lobes instead of the typical three to four.
These distorted neutrophils serve as a hallmark laboratory finding in megaloblastic anemia, directly indicating impaired DNA synthesis caused by your B12 deficiency.
When technicians examine your peripheral blood smear, they’re looking for this critical diagnostic criterion that reflects underlying bone marrow dysfunction.
You’ll typically see these hypersegmented neutrophils accompanied by macrocytosis, where your red blood cells appear larger than normal.
The degree of hypersegmentation often correlates with your deficiency’s severity, providing valuable insight into the extent of megaloblastic changes occurring within your bone marrow.
Hypersegmented Neutrophils as Early Warning Signs
When examining a peripheral blood smear, you’ll notice that hypersegmented neutrophils serve as one of the earliest detectable signs of vitamin B12 deficiency.
These abnormal white blood cells contain more than five nuclear lobes, contrasting sharply with the typical three to four lobes found in healthy neutrophils.
You can use hypersegmented neutrophils as vital diagnostic indicators because they often appear before other symptoms become apparent. Their presence signals impaired DNA synthesis affecting cellular maturation processes.
Key clinical significance includes:
- Early detection of megaloblastic anemia before severe symptoms develop
- Prompt identification of underlying vitamin B12 deficiency requiring immediate intervention
- Prevention of neurological complications through timely diagnosis
You’ll frequently observe these cells alongside macrocytic red blood cells, reinforcing your suspicion of megaloblastic anemia and guiding appropriate treatment decisions.
Ineffective Erythropoiesis and Iron Utilization Issues
While hypersegmented neutrophils provide early detection opportunities, vitamin B12 deficiency simultaneously disrupts the fundamental process of red blood cell production through ineffective erythropoiesis.
Your bone marrow becomes hyperplastic, producing increased numbers of erythroid precursors that can’t mature properly. These megaloblasts fail to synchronize nuclear and cytoplasmic development, creating large, immature cells incapable of effective oxygen transport.
The disrupted DNA synthesis elevates your mean corpuscular volume above 100 fL, marking megaloblastic anemia.
Paradoxically, you’ll experience iron accumulation in your marrow and liver despite having anemia. This occurs because impaired erythropoiesis prevents proper iron utilization, creating a situation where iron builds up even as your body struggles with oxygen-carrying capacity from defective red blood cells.
Recovery Timeline for Bone Marrow After B12 Treatment
Once you begin vitamin B12 treatment, your bone marrow responds remarkably quickly to the restored nutrient levels.
Your bone marrow demonstrates an impressive ability to regenerate healthy blood cells once vitamin B12 levels are restored through proper treatment.
You’ll see significant improvements in hematological parameters within 4 to 8 weeks as your bone marrow produces healthy red blood cells more efficiently.
Your recovery follows a predictable timeline:
- Red blood cell normalization – You’ll observe recovery within 1-2 months, with deficiency anemia resolving as your marrow responds.
- MCV correction – Your mean corpuscular volume normalizes within 4-6 weeks, indicating decreased macrocytic cells.
- Neurological improvement – Your nerve symptoms may take 6 weeks to 3 months for relief.
Regular monitoring of blood counts and vitamin B12 levels guarantees sustained recovery and addresses any underlying absorption issues affecting long-term outcomes.
Monitoring Bone Marrow Response to Supplementation
You’ll need to track specific markers to gauge how well your bone marrow responds to B12 supplementation.
Your reticulocyte count provides the earliest indication of recovery, typically rising within 2-3 days of starting treatment.
Monitoring your complete blood count over 4-8 weeks reveals the broader timeline of bone marrow restoration and helps confirm treatment effectiveness.
Early Response Markers
Though B12 deficiency can take months or years to develop, your bone marrow responds remarkably quickly to proper supplementation.
Within days to weeks, you’ll see encouraging early response markers that indicate your treatment’s working effectively.
Your healthcare provider will monitor several key indicators:
- Reticulocyte count increase – These immature red blood cells rise first, showing your bone marrow’s renewed activity
- Lactate dehydrogenase levels drop – Previously elevated LDH decreases as your bone marrow starts producing healthy red blood cells efficiently
- Peripheral blood smear improvements – Macrocytic cells and hypersegmented neutrophils begin disappearing within weeks
These early response markers appear before your hemoglobin normalizes, giving you and your doctor confidence that supplementation’s restoring proper bone marrow function and cellular production.
Recovery Timeline Assessment
While early response markers provide immediate reassurance, establishing a thorough recovery timeline helps you and your healthcare team track your bone marrow’s complete restoration.
Your recovery timeline typically spans 4 to 8 weeks, during which hematological parameters gradually improve. Within 1 to 2 weeks, you’ll see reticulocyte counts increase, signaling effective red blood cells production.
If you have severe deficiency, monitoring becomes more intensive—expect blood work every 1 to 2 weeks until stabilization occurs. Your macrocytosis will resolve as MCV normalizes alongside red blood cell recovery.
Preventing Irreversible Bone Marrow Damage
Because your bone marrow can suffer permanent damage from prolonged B12 deficiency, acting quickly becomes essential when symptoms first appear.
Megaloblastic changes develop within weeks, causing your body to produce fewer red blood cells and larger-than-normal cell precursors.
You’ll need proactive monitoring if you’re at higher risk:
- Regular blood tests – Schedule vitamin B12 level checks every 3-6 months if you have absorption issues or follow strict vegetarian diets
- Early intervention – Start supplementation immediately when deficiency is detected, using injections or high-dose oral forms
- Sustained treatment – Maintain consistent therapy to prevent recurrence and long-term complications
Your bone marrow function can recover remarkably fast with proper vitamin B12 deficiency treatment, typically showing blood count improvements within 4-8 weeks when you address the problem promptly.
Frequently Asked Questions
How Does Vitamin B12 Deficiency Affect Bone Marrow?
You’ll develop hyperplastic megaloblastic erythropoiesis when you’re B12 deficient. Your bone marrow produces abnormally large red cell precursors called megaloblasts, showing nuclear-cytoplasmic maturation problems that create ineffective erythropoiesis and macrocytic anemia.
What Does B12 Deficiency Do to Blood Cells?
You’ll develop larger-than-normal red blood cells called macrocytes when you’re B12 deficient. Your neutrophils become hypersegmented, and you’ll experience increased cell breakdown, leading to elevated lactate dehydrogenase levels in your bloodstream.
What Happens to the Body if Bone Marrow Doesn’t Produce Enough Blood Cells?
You’ll experience fatigue from anemia, increased infections from low white blood cells, and easy bruising from reduced platelets. This pancytopenia can cause serious organ dysfunction throughout your body.
What Does CBC Look Like With B12 Deficiency?
You’ll see macrocytic anemia with elevated MCV above 100 fL, low hemoglobin below 80 g/L, decreased white blood cells and platelets, plus hypersegmented neutrophils on your blood smear.




Leave a Reply